Dopavision publishes data from the MyopiaX-1 clinical trial at international conference
Dopavision presented the first 6-month clinical data from its proof-of-concept study of MyopiaX, a novel approach designed to slow the progression of myopia in adolescents, at the 19th International Myopia Conference in Sanya, China. This first public presentation, given by Prof. Ian Flitcroft, Fellow of the Royal College of Ophthalmologists and principal investigator of the MyopiaX-1 study, showed data demonstrating the safety of MyopiaX and providing evidence of a clinical impact on myopia progression over six months.
The presentation, titled "MyopiaX-1 - six-month safety and efficacy results to reduce myopia progression: a randomized, controlled, multicenter study", showed data from the first and main phase of the study, in which children with myopia in Europe were either given MyopiaX exclusively or treated in a control group.
Participants in the MyopiaX group (n = 50) showed an average change in axial length of 0.14 mm and spherical equivalent refractive error of -0.19 D from baseline. Children who were treated in the active control group (n = 34) and received lenses with DIMS (Defocus Incorporated Multiple Segments) technology showed a change of 0.08 mm and -0.16 D over the same period. The MyopiaX-1 study was not statistically designed for comparison between groups, such as in a non-inferiority analysis.
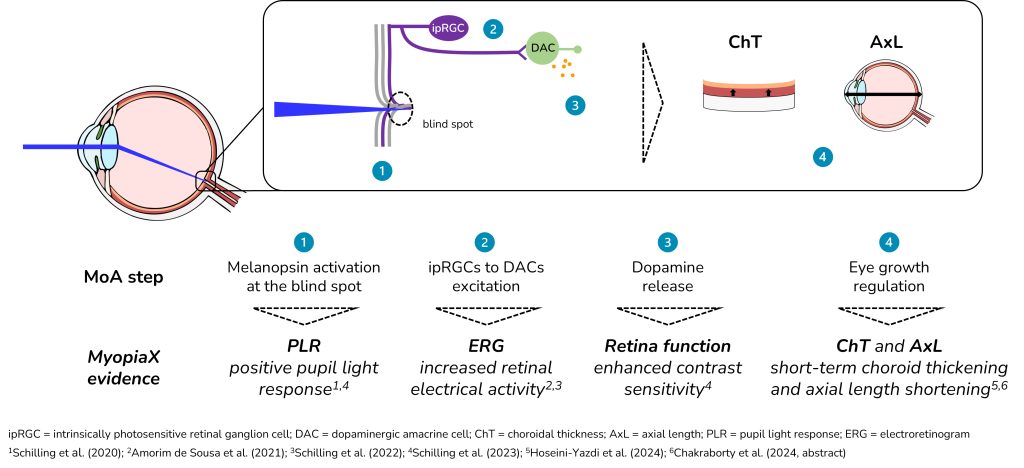
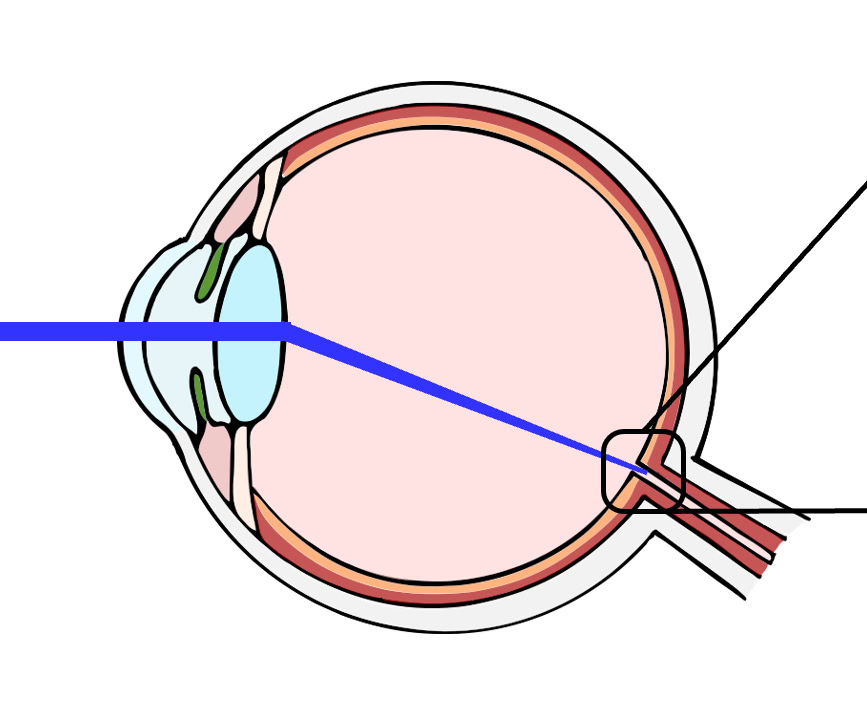
MyopiaX selectively stimulates the blind spot of the eye with light to increase the release of dopamine through melanopsin and slow the progression of myopia. The treatment is delivered via a smartphone application in conjunction with gaming accessories to provide an interactive and easily accessible myopia control solution for children.
"MyopiaX's selective light stimulation technology of the eye is a unique approach," said Prof. Ian Flitcroft, "it is a promising new addition to our toolkit that allows us to tailor treatment to each child's individual profile as their myopia progresses. These initial data from the MyopiaX-1 study help to deepen our understanding of the role of light in slowing myopia."
Mark Wuttke, CEO of Dopavision, adds: "The myopia epidemic is a global problem. public health problem. We are very pleased with these clinical data and see great potential for MyopiaX as a new tool to combat this growing condition. Given the excellent safety profile of MyopiaX and the evidence of its clinical impact on myopia progression over six months, we look forward to advancing this program."
The findings from the full MyopiaX-1 study and further results from the proof-of-concept will be incorporated into future clinical studies and the further development of MyopiaX.